Polymeric inhibitor of Influenza virus


Polymeric inhibitor of Influenza virus


Potent Supramolecule that Change Its Structure on Binding with the Counter Receptor
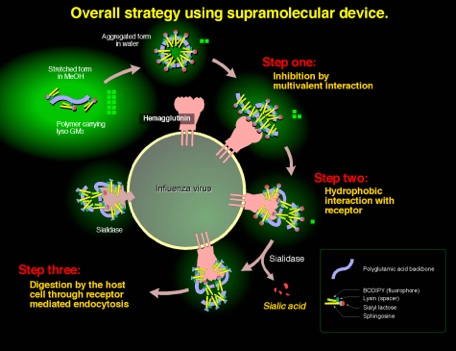
A novel strategy for the inhibition of influenza virus infection by disruption of receptor functions was proposed and described. A polymeric inhibitor that contains lyso GM3 ganglioside was synthesized as amphiphilic polymer with receptor specific ligands. 9-Fluorenylmethyloxylcarbonyl (Fmoc) protected (ε-amino group) L-lysine was attached with the fluorescent tag BODIPY, which was then coupled with lyso GM3 ganglioside to yield a fluorescent GM3 analog. Removal of the Fmoc group followed by amide formation with activated poly-L-glutamic acid (PGA) provided lyso GM3-PGA containing the BODIPY fluorophore. The lyso GM3 content was calculated from the u.v. absorption of the fluorophore to be 3 mol %. Because of the amphiphilic nature, the polymer was shown to have folded structure in aqueous media. The inhibitory activities of lyso GM3-PGA, lyso GM3, and GM3 oligosaccharide (sialyl lactose) against the binding of influenza A/PR/8/34 (H1N1) were investigated using an ELISA assay. The IC50 values for the polymeric inhibitor, GM3, lyso GM3, and sialyllactose were 7.5 x 10-12, 1 x 10-9, 3 x 10-9, and 1.5 x 10-7 mol, respectively, based on monomeric sialic acid. PGA showed no inhibition against the influenza.
Some evidence of the inhibition mechanism
Size of aggregate
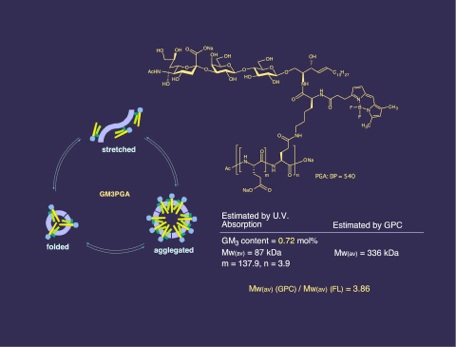
Overall strategy