Polymeric inhibitor of Influenza virus

Polymeric inhibitor of Influenza virus

Potent Supramolecule that Change Its Structure on Binding with the Counter Receptor
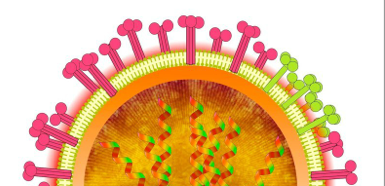
A novel strategy for the inhibition of influenza virus infection by disruption of receptor functions was proposed and described. A polymeric inhibitor that contains lyso GM3 ganglioside was synthesized as amphiphilic polymer with receptor specific ligands. 9-Fluorenylmethyloxylcarbonyl (Fmoc) protected (ε-amino group) L-lysine was attached with the fluorescent tag BODIPY, which was then coupled with lyso GM3 ganglioside to yield a fluorescent GM3 analog. Removal of the Fmoc group followed by amide formation with activated poly-L-glutamic acid (PGA) provided lyso GM3-PGA containing the BODIPY fluorophore. The lyso GM3 content was calculated from the u.v. absorption of the fluorophore to be 3 mol %. Because of the amphiphilic nature, the polymer was shown to have folded structure in aqueous media. The inhibitory activities of lyso GM3-PGA, lyso GM3, and GM3 oligosaccharide (sialyl lactose) against the binding of influenza A/PR/8/34 (H1N1) were investigated using an ELISA assay. The IC50 values for the polymeric inhibitor, GM3, lyso GM3, and sialyllactose were 7.5 x 10-12, 1 x 10-9, 3 x 10-9, and 1.5 x 10-7 molar, respectively, based on monomeric sialic acid. PGA showed no inhibition against the influenza.
Reference
1.Lyso GM3 ganglioside-poly-L-glutamic acid conjugate as inhibitor of influenza hemagglutinin
Kamitakahara, H. Kanie, O. Wong, C,-H.
Angew. Chem. Int. Ed., 1998, 37, 1524-1528.
Some evidence of the inhibition mechanism
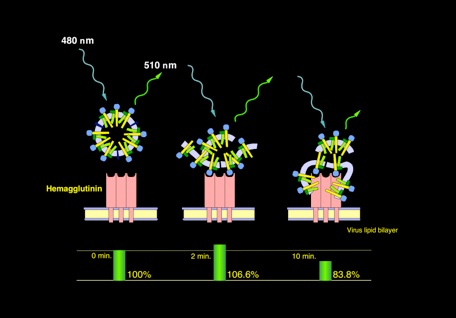
During the inhibition assay, the fluorescence property of the polymer changed indicating the conformational change.
Size of aggregate
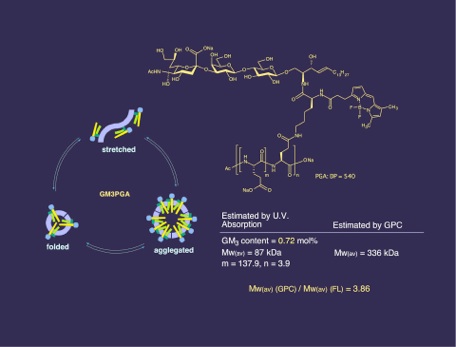
The estimated size (GPC) of the polymer was larger than the synthesized polymer. According to this, it was suggested average of 3.86 monomers were aggregated in aqueous solution.
Overall strategy
It is expected that the polymeric supramolecule might also act on sialidase as well in a same manner, and the effect might persist even after some or complete hydrolysis of sialic acid residues if it occurs after the binding.