Orthogonal glycosylation


Orthogonal glycosylation


Chapter 3
Introducing orthogonal glycosylation and a demonstration in the synthesis of chitooligosaccharide
Kanie, O.; Ito, Y.; Ogawa, T. J. Am. Chem. Soc., 1994, 116, 12073-12074.
In the initial attempt to demonstrate the feasibility of the orthogonal strategy, N-phthaloyl (Phth) protected glucosamine (GlcN) derivatives were chosen as the monosaccharide units. This decision was based solely on the assumption that any stereochemical ambiguity could be eliminated by the strong 1,2-trans-directing nature of the NPhth group. [8] However, it is to be stressed that the basic principle should be applicable to a wide variety of oligosaccharide structures. In addition, the biological significance of β-1,4 linked oligomers of glucosamine (e.g. chitin) are well recognized. [9] Also, the hydroxyl group at the C-4 position of GlcN is known to be relatively less reactive. [10] Therefore, the construction of this type of oligosaccharide is a challenging task.
In order to assess the orthogonality of the aforementioned combination of reactions, the following experiments were at first performed.
Section 1: High Yield Transformations of Thiophenyl Glycoside and Fluoride into The Corresponding Fluoride and The Thioglycoside.
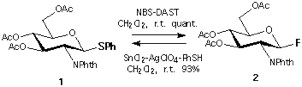
Section 2: An Experiment to Show Chemical Distinctness of Each Reaction.
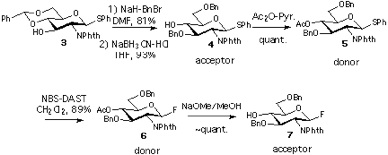
Required GlcN derivatives 4, 5, 6 and 7 were synthesized as shown in the first panel.
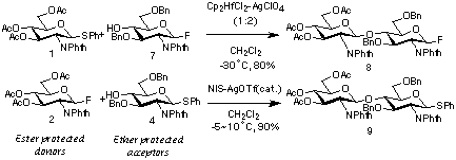
Section 3: Synthesis of Heptasaccharide from Two Suitably Protected Monosaccharide Units.
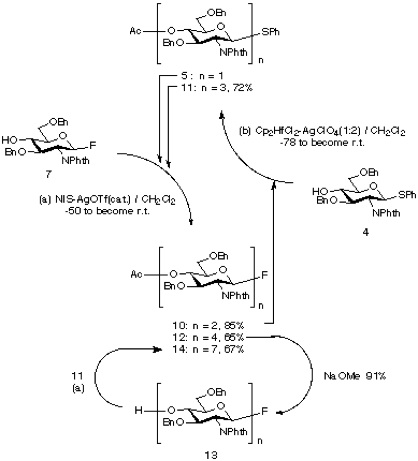
First, thioglycoside donor 5 was coupled with acceptor fluoride 7 under condition (a) to give disaccharide fluoride 10 (85%) which was then reacted with the acceptor 4 to produce 11 [condition (b), 72%]. Subsequent reaction of 11 with 7 [condition (a), 65%] gave tetrasaccharide 12. Having accomplished the stepwise synthesis of a tetrasaccharide, we next examined a block condensation approach. Tetrasaccharide acceptor 13, prepared by Zemplen deacetylation of 12, was coupled with its precursor 11 to give compound 14 [condition (a), 67%], which is again ready for further use as a oligosaccharide donor.


Chap. 1:
Chap. 2:
Chap. 3:
Chap. 4:
Chap. 5:
Chap. 6:

